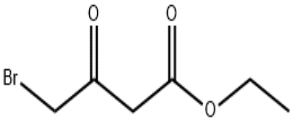Pharmaceutical intermediates industry development trends and development characteristics, opportunities and challenges
1.The development trend of pharmaceutical intermediates industry
The development of fine chemicals is one of the solid foundations of China's construction of a manufacturing power, but also to achieve the national energy saving and emission reduction targets, the development of a low-carbon economy is an important path. Fine chemical industry production process is complex, the production of raw materials and products involving hazardous chemicals, safety and environmental risks are high. In addition, enterprises are also facing many pressures such as the global economic downturn, rising domestic labor costs, and intensified market competition. Pharmaceutical intermediates include antimicrobial drug intermediates, antipyretic and analgesic drug intermediates, cardiovascular drug intermediates and anticancer drug intermediates, etc. Among them, antimicrobial drug intermediates can be divided into quinolones, β-lactams, aminoglycosides, tetracyclines, amidinols, macrolides and so on according to the chemical structure of the antimicrobial drugs synthesized in the further reaction and the mechanism of bacterial inhibition.
1.1 Continuous transfer of pharmaceutical industry to Asian region
The pharmaceutical industry in developed countries in Europe and the United States started earlier, but with the continuous development of global productivity and the deepening of the international division of labor, pharmaceutical companies in developed countries in Europe and the United States are focusing on the research and development of innovative medicines and market development, so that the pharmaceutical intermediates industry is accelerating the transfer of Asian countries, including China and India. In China, for example, with the mature petrochemical industry chain, rich basic resources, so that the production of pharmaceutical intermediates required by the various types of major raw and auxiliary materials in the country can be obtained, improve production efficiency and reduce transportation costs; at the same time, relying on the domestic perfect industrial system, so that the domestic enterprises in the production of equipment, building construction, etc. cost and quality of the competitive advantage, to speed up the production of products; coupled with the enterprises in China, Research institutes in China have been in the field of pharmaceuticals, fine chemicals have many years of technical accumulation, the formation of intellectual property rights with core competitiveness of the system, training a large number of first-class R & D personnel and industrial workers to ensure that the production line of pharmaceutical intermediates can run smoothly, the production process can be continuous progress.
Therefore, under the trend of deepening international division of labor, the center of gravity of the pharmaceutical intermediates industry is expected to further shift from Europe and the United States to the Asian region, and the scale of China's pharmaceutical intermediates industry will be further expanded to form an industry pattern dominated by Asia, which will not only satisfy the needs of the development of China's pharmaceutical industry, but also provide key pharmaceutical intermediates for the global manufacturers of APIs, generic drugs and innovative drugs and promote the development of the world's pharmaceutical industry. Pharmaceutical industry development.
1.2 Increasing value-added products and process complexity
The synthesis of medicine depends on high-quality pharmaceutical intermediates. With the pharmaceutical industry's technological innovation, industrial innovation and upgrading, the pharmaceutical intermediates will also put forward higher requirements. Although the development prospects of China's pharmaceutical intermediates, but at present the degree of development of China's pharmaceutical intermediates industry and the needs of the pharmaceutical industry there is still a certain gap, some products require a high level of technology, the domestic can not organize the production of the basic dependence on imports, there are also a number of products, although in the output of the domestic pharmaceutical industry to meet the requirements of the higher cost, quality is not acceptable, affecting the competitiveness of pharmaceutical products, it is still necessary to Improve the production process.
In order to meet the continuous development of the pharmaceutical industry, China's pharmaceutical intermediates industry will be from the original low level of repetition, lack of innovation, the traditional mode of crude production to innovation and upgrading, high quality, high level of direction, with the advancement of R & D and development, the accumulation of independent intellectual property rights, pharmaceutical intermediates of the added value of the product and the complexity of the process will be continuously improved, become a strong guarantee for the development of the pharmaceutical industry.
1.3 Energy saving and environmental protection requirements are becoming increasingly stringent
In recent years, the state vigorously advocate the green and low-carbon development of the pharmaceutical industry chain, improve the level of green manufacturing, the implementation of the pharmaceutical industry carbon emission reduction action to build a green industrial system; at the same time, the state of the chemical industry has implemented a rigorous environmental protection inspections and rectification of energy-saving and environmentally friendly processes in the industry has put forward stringent requirements, eliminating a number of technologically weak, poor product quality of the small enterprises and workshop-type factories, and promote the industry to environmental protection, formalization direction. The industry has been prompted to develop in the direction of environmental protection and formalization.
With the increasingly stringent requirements of energy conservation and environmental protection, coupled with the complexity of the pharmaceutical intermediates manufacturing process, the reaction link is more, so the industry manufacturers need to vigorously develop green advanced technology, with environmental protection facilities in line with the norms of the treatment facilities and low-consumption and energy-saving production equipment, in order to implement the goal of reducing consumption and emission reduction responsibilities.
1.4The degree of automation continues to improve
In order to meet the needs of a wide variety of complex structure of the production of drugs, pharmaceutical intermediates are usually complex, more reaction steps, in the past, most of the production enterprises follow the traditional production process, for the application of new technologies is still little, most of the production process is manual, automation is not yet commonly used, resulting in poor product quality, low production efficiency, and safety is difficult to protect.
With the national supporting system for production safety continues to improve, labor costs continue to rise, the pharmaceutical industry on the quality of intermediates more and more stringent requirements, so that the pharmaceutical intermediates of the production process automation is increasingly high, which requires manufacturers of pharmaceutical intermediates for the production characteristics of the design of automated control programs, and automatic control instrumentation selection, and constantly improve the degree of automation of the production industry.
2. Pharmaceutical intermediates industry characteristics
Pharmaceutical intermediates are chemical raw materials to APIs or pharmaceutical production process of fine chemical products, usually highly technology-intensive, high value-added specialty chemicals. Due to the many varieties of pharmaceuticals, complex chemical structure, so the demand for more varieties of intermediates. Unlike large-scale chemical production processes, the production process of pharmaceutical intermediates is usually characterized by miniaturization, single-batch intermittent and multi-functionality; some pharmaceutical intermediates manufacturers have developed relatively large-scale, automated, continuous and modular production processes by combining the experience of large-scale chemical production. The upstream industry of pharmaceutical intermediates is basic chemical raw materials, and the downstream industry is chemical APIs and chemical drug preparations.
Pharmaceutical intermediates industry is an important link in the industry chain of the pharmaceutical industry, with the added value of China's pharmaceutical intermediates and the increasing complexity of the process, is becoming a strong guarantee for the development of the pharmaceutical industry. Pharmaceutical intermediates include antimicrobial drug intermediates, antipyretic and analgesic drug intermediates, cardiovascular drug intermediates and anticancer drug intermediates, etc. Among them, antimicrobial drug intermediates can be classified into quinolones, β-lactams, aminoglycosides, tetracyclines, amidinols, macrolides and so on in accordance with the chemical structure of the further reaction of the synthesized antimicrobial drugs and the bacterial inhibition mechanism.
China's pharmaceutical intermediates industry after years of considerable development, China's pharmaceutical production needs have been basically by the production of pharmaceutical intermediates to meet, but there are still a small number of synthesis technology complex high-end intermediates need to be imported to meet. China now benefits from talent, patent protection, infrastructure and cost structure and other aspects of the obvious competitive advantage, has become one of the world's major R & D and production base of pharmaceutical intermediates, not only for generic drug manufacturers to provide a large number of high-quality pharmaceutical intermediates, but also increasingly become a preferred choice of pharmaceutical companies to strategically cooperate with a large number of manufacturers of APIs, generics and innovative drugs, such as the provision of key pharmaceutical intermediates for a large number of manufacturers of APIs, generics and innovative drugs.
3. Pharmaceutical intermediates industry opportunities
3.1 The scale of industry demand continues to expand, the application field of the product continues to extend
Pharmaceutical industry is a strategic industry related to national livelihood, economic development and national security, and is an important foundation for a healthy China. China's pharmaceutical industry is in a stage of vigorous development, antibacterial drugs are an important part of the pharmaceutical industry. With the growth of the total global population and the acceleration of population aging, the demand for antimicrobial drugs is steadily rising, driving the industry to expand the scale of demand.
3.2 National industrial policy support, industry standardization and orderly development
In recent years, the state attaches great importance to the sustained and stable development of the industry, and has issued the "China's National Economic and Social Development 14th Five-Year Plan and 2035 Vision Outline," "14th Five-Year Plan" Pharmaceutical Industry Development Plan, "14th Five-Year Plan" National Pesticides Development Plan for Pharmaceutical Industry", "14th Five-Year Plan", "14th Five-Year Plan", "National Pesticide Industry Development Plan", "Implementation Plan for Promoting the High-Quality Development of API Industry" and other industry development plans and industrial policy guidelines, effectively promote the industry's industrial restructuring and optimization and upgrading, support the standardization of enterprises, elimination of backward production capacity, improve industrial concentration, improve the competitive environment, to guide the industry to standardize the development of an orderly, enhance the core competitiveness of the leading enterprises to achieve the industry's sustainable scientific development for the Pharmaceutical intermediates industry to create a good policy environment for the long-term stable development.
3.3 Development opportunities brought by the transfer of the global pharmaceutical intermediates industry
Under the trend of high operating costs and saturated labor supply in developed countries, and the increasingly mature and perfect industrial chain in developing countries and regions, the global intermediates industry is accelerating its transfer to Asian countries including China and India. China's rapid development of the market, but also has sufficient professional and technical personnel, rich basic resources, petrochemical industry chain is perfect and other advantages, in the international division of labor system is deepening the general trend, China's relevant industry scale will be further expanded, the formation of Asia-led industrial pattern, not only to meet the needs of China's industrial development, but also for the global market to provide the key intermediates products for the pharmaceutical intermediates industry to bring Further development opportunities for the pharmaceutical intermediates industry.
3.4 The introduction of policies related to healthcare reform "band purchasing".
In November 2018, the Fifth Meeting of China's Comprehensively Deepening Reform Committee considered and passed the Pilot Program for State-Organized Centralized Purchasing of Pharmaceuticals, specifying that "state-organized, alliance-purchasing and platform-operated centralized purchasing of pharmaceuticals" is a "state-organized, alliance-purchasing and platform-operated pilot program for state-organized centralized purchasing of pharmaceuticals". The Pilot Program on Centralized Purchasing of Medicines by State Organization was adopted at the Fifth Meeting of China's Comprehensive Deepening Reform Committee in November 2018, which clearly defines the general idea of "state-organized, alliance purchasing and platform operation" for band-quantity procurement.
The policy of banded purchasing aims to explore the reasonable pricing of clinical medicines on the premise of ensuring the consistency of the quality of medicines and the purchasing volume, downplaying the influence of academic promotion, so as to effectively reduce the price of clinical medicines while guaranteeing the economic interests of successful bidding enterprises. First of all, antibacterial drugs, as one of the main drugs used in China's public hospitals, have long been widely used and have existed for a long time in the health insurance catalog, and the band purchasing policy will not have a significant adverse impact on the number of antibacterial drugs used; secondly, the substantial reduction in the terminal drug price mainly reduces the sales expenses of the preparation manufacturers; lastly, in order to ensure that the scale and quality of raw material supply up to the standard, downstream preparations and raw material drug manufacturers will Finally, in order to ensure the scale and quality of raw material supply, downstream preparation and API manufacturers will choose intermediate enterprises with stable production capacity and quality as long-term partners, so that high-quality upstream intermediate enterprises in the industry chain have improved their status and retain a certain bargaining power. Therefore, the introduction of healthcare reform "band purchasing" related policies will help promote the operation of standardized pharmaceutical intermediates manufacturers and downstream preparations, API manufacturers to work closely together, bringing opportunities for the development of pharmaceutical intermediates industry.
4. Challenges faced by the industry
4.1 Impact of national policies to regulate the use of antimicrobial drugs
With the introduction of a series of policies to regulate the use of antimicrobial drugs, such as "Measures for the Management of Clinical Application of Antimicrobial Drugs" and "National Veterinary Antimicrobial Drugs Use Reduction Action Program (2021-2025)", on the one hand, the use of antimicrobial drugs has been subject to stricter supervision and management; on the other hand, the introduction of relevant policies also helps to promote scientific, rational and standardized use of antimicrobial drugs. On the one hand, the use of antibacterial drugs is subject to more stringent supervision and management; on the other hand, the introduction of relevant policies also helps promote the scientific, rational and standardized use of antibacterial drugs, prolonging the life cycle of antibacterial drugs, which is conducive to the long-term sound development of the industry.
4.2 Energy saving and environmental protection requirements are tightening
In recent years, the state has issued the "14th Five-Year Plan" for the development of the pharmaceutical industry, "14th Five-Year Plan" for the development of the national pesticide industry, "Comprehensive List of Environmental Protection (2021 Edition)," "to promote the green development of the API industry's guiding opinions" and other advocates of energy-saving and low-carbon development of the industry, and improve the green development of the industry. Industry energy-saving and low-carbon development, improve the level of green manufacturing policy sound, energy-saving and environmental protection requirements of industry enterprises are becoming increasingly stringent, on the one hand, a large number of backward technology, technologically weak enterprises gradually be eliminated, on the other hand, but also prompted the industry to further enhance the degree of concentration of the market resource allocation to the overall strength of the stronger, energy-saving and environmentally friendly technology leading the industry head of the enterprise aggregation.
4.3 Pharmaceutical intermediates industry part of the enterprise capital strength is weak
The development and technological progress of the pharmaceutical intermediates industry requires a lot of R & D investment, whether it is new technology, new product development and transformation, need financial support, but in addition to a few large enterprises, the industry is mostly small and medium-sized enterprises, in the financial strength of a certain degree of inadequacy, and its main source of funds for their own business accumulation and bank loans, in the scale of funds and source channels are a certain degree of insufficiency , limiting the scientific and technological innovation of enterprises in the industry.